MODEL 54eA SECTION 8.0
CALIBRATION - FREE CHLORINE
SECTION 8.0
CALIBRATION - FREE CHLORINE
8.1 INTRODUCTION
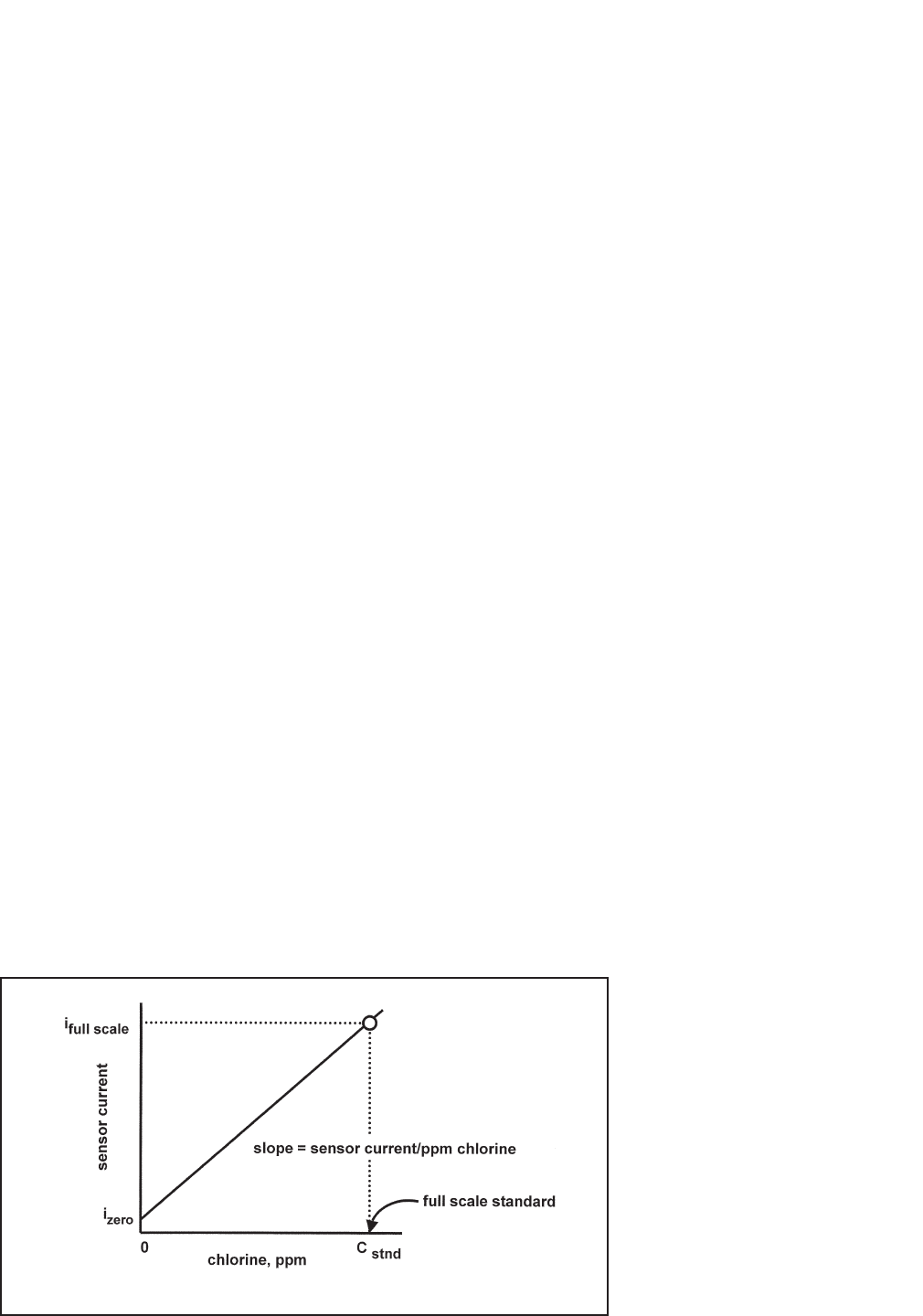
As Figure 8-1 shows, a free chlorine sensor generates a current directly proportional to the concentration of free
chlorine in the sample. Calibrating the sensor requires exposing it to a solution containing no chlorine (zero stan-
dard) and to a solution containing a known amount of chlorine (full-scale standard).
The zero standard is necessary because chlorine sensors, even when no chlorine is in the sample, generate a
small current called the residual current. The controller compensates for the residual current by subtracting it from
the measured current before converting the result to a chlorine value. New sensors require zeroing before being
placed in service, and sensors should be zeroed whenever the electrolyte solution is replaced. Either of the fol-
lowing makes a good zero standard:
• Deionized water containing about 500 ppm sodium chloride. Dissolve 0.5 grams (1/8 teaspoonful) of table
salt in 1 liter of water. DO NOT USE DEIONIZED WATER ALONE FOR ZEROING THE SENSOR. THE
CONDUCTIVITY OF THE ZERO WATER MUST BE GREATER THAN 50 μS/cm.
• Tap water known to contain no chlorine. Expose tap water to bright sunlight for at least 24 hours.
The purpose of the full-scale standard is to establish the slope of the calibration curve. Because stable chlorine
standards do not exist, the sensor must be calibrated against a test run on a grab sample of the process liq-
uid. Several manufacturers offer portable test kits for this purpose. Observe the following precautions when tak-
ing and testing the grab sample.
• Take the grab sample from a point as close to the sensor as possible. Be sure that taking the sample does
not alter the flow of the sample to the sensor. It is best to install the sample tap just downstream from the
sensor.
• Chlorine solutions are unstable. Run the test immediately after taking the sample. Try to calibrate the sen-
sor when the chlorine concentration is at the upper end of the normal operating range.
Free chlorine measurements made with the 499ACL-01 sensor also require a pH correction. Free chlorine is the sum of
hypochlorous acid (HOCl) and hyprochlorite ion (OCl
-
). The relative amount of each depends on the pH. As pH increas-
es, the concentration of HOCl decreases and the concentration of OCl
-
increases. Because the sensor responds only
to HOCl, a pH correction is necessary to properly convert the sensor current into a free chlorine reading.
The controller uses both automatic and manual pH correction. In automatic pH correction, the controller continu-
ously monitors the pH of the solution and corrects the free chlorine reading for changes in pH. In manual pH cor-
rection, the controller uses a fixed pH value entered by the user to make the correction. Generally, if the pH
changes more than about 0.2 units over short periods of time, automatic pH correction is best. If the pH is rela-
tively steady or subject only to seasonal changes, manual pH correction is adequate.
During calibration, the controller must know the pH of the sample. If the controller is using automatic pH correc-
tion, the pH sensor (properly calibrated) must be in the process liquid before starting the calibration. If the
controller is using manual pH correction, be sure to enter the pH value before starting the calibration.
The Model 499ACL-01 free chlorine
sensor loses sensitivity at high con-
centrations of chlorine. The 54eA
controller has a dual slope feature
that allows the user to compensate
for the non-linearity of the sensor.
However, for the vast majority of
applications, dual slope calibration
is unnecessary.
FIGURE 8-1. Sensor Current as a Function of Free Chlorine Concentration
55


















