MODEL 54eA SECTION 7.0
CALIBRATION - DISSOLVED OXYGEN
SECTION 7.0
CALIBRATION - DISSOLVED OXYGEN
7.1 INTRODUCTION
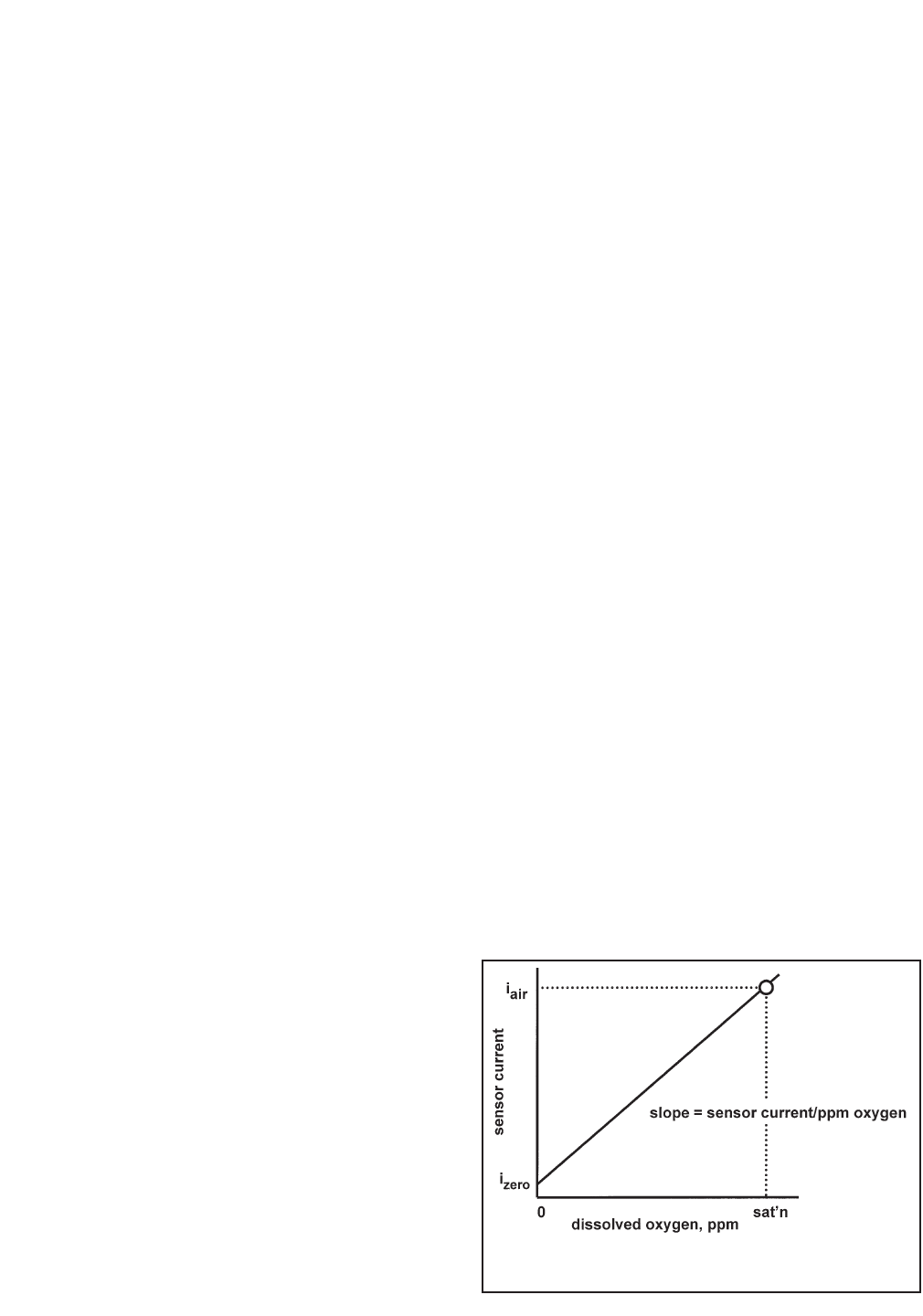
As Figure 7-1 shows, oxygen sensors generate a current directly proportional to the concentration of dissolved
oxygen in the sample. Calibrating the sensor requires exposing it to a solution containing no oxygen (zero stan-
dard) and to a solution containing a known amount of oxygen (full-scale standard).
The zero standard is necessary because oxygen sensors, even when no oxygen is present in the sample, gener-
ate a small current called the residual current. The analyzer compensates for the residual current by subtracting it
from the measured current before converting the result to a dissolved oxygen value. New sensors require zeroing
before being placed in service, and sensors should be zeroed whenever the electrolyte solution is replaced. The
recommended zero standard is 5% sodium sulfite in water, although oxygen-free nitrogen can also be used.
The Model 499A TrDO sensor, used for the determination of trace (ppb) oxygen levels, has very low resid-
ual current and does not normally require zeroing. The residual current in the 499A TrDO sensor is equivalent
to less than 0.5 ppb oxygen.
The purpose of the full-scale standard is to establish the slope of the calibration curve. Because the solubility of
atmospheric oxygen in water as a function of temperature and barometric pressure is well known, the natural
choice for a full-scale standard is air-saturated water. However, air-saturated water is difficult to prepare and use,
so the universal practice is to use air for calibration. From the point of view of the oxygen sensor, air and air-sat-
urated water are identical. The equivalence comes about because the sensor really measures the chemical poten-
tial of oxygen. Chemical potential is the force that causes oxygen molecules to diffuse from the sample into the
sensor where they can be measured. It is also the force that causes oxygen molecules in air to dissolve in water
and to continue to dissolve until the water is saturated with oxygen. Once the water is saturated, the chemical
potential of oxygen in the two phases (air and water) is the same.
Oxygen sensors generate a current directly proportional to the rate at which oxygen molecules diffuse through a
membrane stretched over the end of the sensor. The diffusion rate depends on the difference in chemical poten-
tial between oxygen in the sensor and oxygen in the sample. An electrochemical reaction, which destroys any oxy-
gen molecules entering the sensor, keeps the concentration (and the chemical potential) of oxygen inside the sen-
sor equal to zero. Therefore, the chemical potential of oxygen in the sample alone determines the diffusion rate
and the sensor current.
When the sensor is calibrated, the chemical potential of oxygen in the standard determines the sensor current.
Whether the sensor is calibrated in air or air-saturated water is immaterial. The chemical potential of oxygen is the
same in either phase. Normally, to make the calculation of solubility in common units (like ppm DO) simpler, it is
convenient to use water-saturated air for calibration.
Automatic air calibration is standard. The user simply exposes the sensor to water-saturated air. The controller
monitors the sensor current. When the current is stable, the controller stores the current and measures the baro-
metric pressure and temperature. The temperature element is part of the dissolved oxygen sensor. The pressure
sensor is inside theanalyzer. From the temperature, the
controller calculates the saturation vapor pressure of
water. Next, it calculates the pressure of dry air by sub-
tracting the vapor pressure from the barometric pressure.
Using the fact that dry air always contains 20.95% oxy-
gen, the analyzer calculates the partial pressure of oxy-
gen. Once the analyzer knows the partial pressure of oxy-
gen, it uses the Bunsen coefficient to calculate the equi-
librium solubility of atmospheric oxygen in water at the
prevailing temperature. At 25°C and 760 mm Hg, the
equilibrium solubility is 8.24 ppm.
Often it is too difficult or messy to remove the sensor from
the process liquid for calibration. In this case, the sensor
can be calibrated against a measurement made with a
portable laboratory instrument. The laboratory instrument
typically uses a membrane-covered amperometric sensor
that has been calibrated against water-saturated air.
FIGURE 7-1. Sensor Current as a Function of
Dissolved Oxygen Concentration
49


















