– 12 –
FILE NO. SVM-03008
(2) Characteristics required for flux
• Activated temperature of flux coincides with the
brazing temperature.
• Due to a wide effective temperature range, flux
is hard to carbonize.
• It is easy to remove slag after brazing.
• The corrosive action to the treated metal and
brazing filler is minimum.
• It excels in coating performance and is
harmless to the human body.
As the flux works in a complicated manner as
described above, it is necessary to select an
adequate type of flux according to the type and
shape of treated metal, type of brazing filler and
brazing method, etc.
(3) Types of flux
• Noncorrosive flux
Generally, it is a compound of borax and boric
acid.
It is effective in case where the brazing
temperature is higher than 800°C.
• Activated flux
Most of fluxes generally used for silver brazing
are this type.
It features an increased oxide film removing
capability due to the addition of compounds
such as potassium fluoride, potassium chloride
and sodium fluoride to the borax-boric acid
compound.
(4) Piping materials for brazing and used brazing
filler/flux
Piping Used brazing Used
material filler flux
Copper - Copper Phosphor copper Do not use
Copper - Iron Silver Paste flux
Iron - Iron Silver Vapor flux
2-5-3. Brazing
As brazing work requires sophisticated techniques,
experiences based upon a theoretical knowledge, it
must be performed by a person qualified.
In order to prevent the oxide film from occurring in the
pipe interior during brazing, it is effective to proceed
with brazing while letting dry Nitrogen gas (N
2
) flow.
Never use gas other than Nitrogen gas.
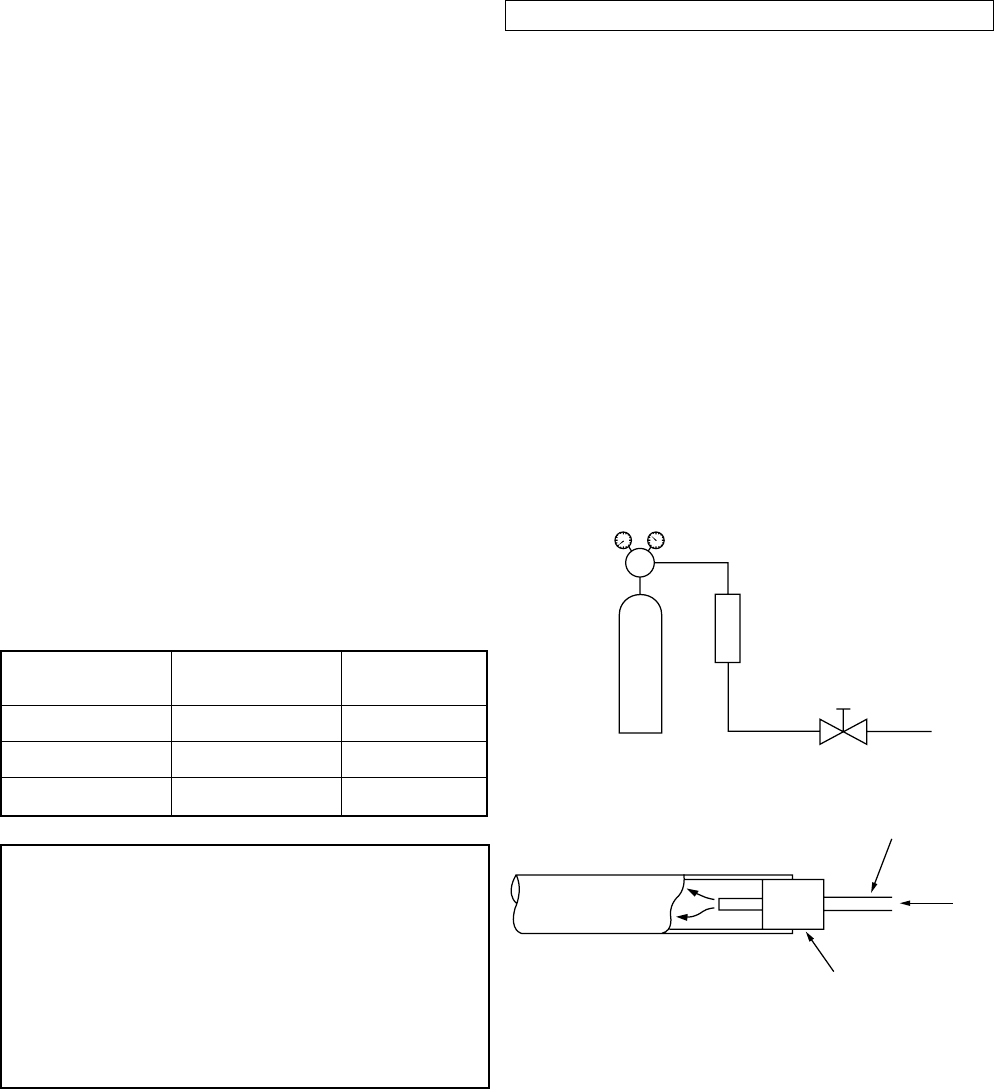
(1) Brazing method to prevent oxidation
1 Attach a reducing valve and a flow-meter to the
Nitrogen gas cylinder.
2 Use a copper pipe to direct the piping material,
and attach a flow-meter to the cylinder.
3 Apply a seal into the clearance between the
piping material and inserted copper pipe for
Nitrogen in order to prevent backflow of the
Nitrogen gas.
4 When the Nitrogen gas is flowing, be sure to
keep the piping end open.
5 Adjust the flow rate of Nitrogen gas so that it is
lower than 0.05 m
3
/Hr or 0.02 Mpa (0.2 kgf/
cm
2
) by means of the reducing valve.
6 After performing the steps above, keep the
Nitrogen gas flowing until the pipe cools down
to a certain extent (temperature at which pipes
are touchable with hands).
7 Remove the flux completely after brazing.
Fig. 2-5-1 Prevention of oxidation during brazing
1 Do not enter flux into the refrigeration cycle.
2 When chlorine contained in the flux remains
within the pipe, the lubricating oil deteriorates.
Therefore, use a flux which does not contain
chlorine.
3 When adding water to the flux, use water which
does not contain chlorine (e.g. distilled water or
ion-exchange water).
4 Remove the flux after brazing.
Nitrogen gas
cylinder
M
Flow meter
Stop valve
From Nitrogen cylinder
Nitrogen
gas
Rubber plug
Pipe


















